- Products
- Face Shield (PPE)
- Face shields are an important part of personal protection equipment (PPE), preventing airborne droplets from reaching the wearer’s face. Used by the NHS and in other clinical settings in the UK.
- Double Sided Materials
- Double sided materials are replacing traditional fixing methods in a range of industries and applications. They also offer additional functionality such as sealing, damping and conducting.
- Double Sided Tapes
- VHB™ Tapes
- Adhesive Transfer Tapes
- Foam Tapes
- Medical Tapes
- Heat Activated Films
- Electrically Conductive Tapes
- Thermal Tapes
- Ultra Clean, Low Outgassing Tapes
- Optically Clear Tapes
- Differential Tapes
- Reclosable fasteners
- Single Sided Materials
- Attach, seal, mark, mask and protect with our extensive range of single coated tapes, foams, films and foils.
- Foam Tapes
- Foil Tapes
- Cloth Tapes
- Protection Films
- Masking Films and Tapes
- RFI and EMI Shielding Tapes
- Plastic Film Tapes
- Single Sided Medical Tapes
- Medical grade materials
- Our range of medical grade materials offer unique characteristics for stick to skin applications and can be sterilised by EtO, gamma and steam. Other products include speciality films and foils for a range of medical applications.
- Double Sided Medical Tapes
- Single Sided Medical Tapes
- Nonwoven and Woven Medical Tapes
- Medical Grade Foam
- Medical Grade Film
- Medical Grade Foil
- Silicone Medical Tapes
- Hydrocolloid Tapes
- Reclosable Fasteners
- Foams and Rubbers
- We offer coated and non-coated foam and rubber materials for a variety of applications including gasketing, sealing, vibration and sound dampening and energy absorption.
- Medical Grade Foam
- Neoprene Foam
- Polyurethane foam
- VHB™ Tapes
- Polyethylene Foam
- Polyester Foam
- Silicone Foam
- Silicone Rubber
- Polyurethane Rubber
- Conductive Elastomers
- 3M™ Bumpons™
- PVC Foam
- Electrical Tapes
- Our electrical tapes come in a variety of backings and adhesives to meet industry specifications including military, UL and CSA.
- Polyester Tapes
- Epoxy Tapes
- Polyimide Tapes
- PTFE Tapes
- Vinyl Tapes
- Paper Tapes
- Glass Cloth Tapes
- Acetate Cloth Tapes
- Antistatic Tapes
- Filament Reinforced Tapes
- Composite Film Tapes
- Non-Adhesive Materials
- Self Amalgamating Tape
- RFI and EMI Shielding
- We offer a selection of the most versatile and satisfactory RFI and EMI shielding solutions, for effective shielding of wirelessly transmitting devices.
- RFI and EMI Shielding Tapes
- Electrically Conductive Tapes
- Conductive Elastomers
- Metallised Fabric Materials
- Microwave Absorbing Materials
- Fabric over Foam
- Knitted Wire Mesh
- Fingerstock
- Spring Contacts
- Board Level Shields
- Vent Panels
- Thermal Management
- As electronics become smaller and more powerful, the need for effective thermal materials increases. We offer a variety of products to suit most applications.
- Thermally Conductive Tapes
- Thermally Gap Pads
- Thermal Interface Phase Change Material
- Electrically Conductive Thermal Interface Materials
- Thermally Conductive Electrical Insulators
- Thermal Grease
- Venting and Filtration Materials
- For the management of temperature, humidity, gases and pressure, and the prevention of liquid, dust and chemical contamination. We hold an expanding range of materials to suit your every application.
- Non-Adhesive Vent Material
- Vent Discs
- Automation
- Automating the application of the adhesive components results in faster and more cost-effective manufacturing.
- Face Shield (PPE)
- Industries
- Healthcare
- We offer our customers access to a superior range of medical grade materials for the varied applications found within the healthcare market. Our commitment to quality, demonstrated by our ISO 13485 registration, FDA registration and ISO Class 7 Clean Room capability means our customers are confident in the superior quality we can achieve for their medical products.
- Ostomy
- Diagnostics
- Wound Care
- Medical Device Assembly
- Device Stick to Skin
- Cosmetic Products
- Optical
- Automotive
- We fully understand the key needs of the automotive market; we offer quality management to ISO 9001, clean room manufacturing and TS16949 compliance. This, combined with our access to World leading materials, makes us the first choice partner in the manufacture of automotive components.
- Automotive Electronics
- Automotive Bodies and External Accessories
- Automotive Interior Parts and Accessories
- EV Batteries
- Electronics
- We supply adhesive components into a range of electronics applications, from bonding of dissimilar substrates, to sealing, cushioning, shielding and insulating of components. Whatever your needs, our access to a variety of products, and the ability to create bespoke constructions and components, will ensure we have a solution to your unique requirements.
- Consumer Electronics
- Domestic Appliances
- Rugged Devices
- Industrial
- Industrial grade materials offer strength and resilience, as well as resistance to UV, chemicals, oil and extreme temperatures. Whatever your needs, our experts can advise on the optimum product for your application. We can convert your material into bespoke components for enhanced manufacturing efficiencies and cost savings.
- Life Safety
- The safety critical applications of this market require trusted adhesive solutions to ensure high quality and reliability of products. We offer a range of materials for bonding, sealing, insulating, shielding and venting for a range of detection, monitoring and emergency devices. Whatever your needs, we have the experience, materials and converting expertise to offer the optimum solution to the unique requirements of your products and processes.
- Fire, Smoke and Gas Detection
- Emergency and Security
- Personal Safety
- Military & Defence
- We offer a range of specialist products and manufacturing processes to satisfy the safety critical needs of the defence industry. With Quality Management accreditation to ISO 9001, Clean Room manufacturing and SC21 accreditation, we are confident in meeting the quality expectations of our military and defence customers.
- Aerospace
- Adhesive tapes offer a range of trusted solutions for aerospace applications and are commonly used for bonding and sealing, as well as vibration dampening, shielding, cooling, masking and venting. We have access to a vast range of materials, which we can convert into bespoke components for enhanced quality and manufacturing efficiencies.
- Building Interiors
- We offer a range of bonding tapes and films for the assembly and protection of furniture, partition walls, flooring systems, HVAC products, doors and windows. Whether you require a permanent bonding solution or temporary protection, we can advise on the optimum product for your unique application.
- Flooring
- Furniture
- HVAC
- Doors and Windows
- Partition Walls
- Lighting
- Modern lighting utilises many adhesive components to enhance the aesthetics and the efficiency of lighting products. We provide bespoke adhesive components to efficiently bond, seal, vent, dissipate heat and reflect light in a range of lighting applications.
- Renewable Energy
- Solar and wind energy products are subject to the harshest external environments throughout their lifetime. This includes extreme temperatures, UV light, wind, rain and debris. We offer a range of performance materials to protect components and resist these environmental extremes, as well as efficiently bond, insulate and vent internal components.
- Solar Energy
- Wind Energy
- Healthcare
- Capabilities
- About Us
- Why Parafix
- We have over 50 years experience in converting tapes and flexible materials for a diverse range of industries and applications. During this time we have built long lasting relationships with suppliers and customers and have built on our experience, capabilities and our reach throughout Europe and beyond.
- We add value
- Experience
- Reach
- Unique customer relationships
- Unique supplier relationships
- First class facilities
- Quality
- From design conception to final production and assembly, quality is paramount in providing our customers with products that consistently reach their desired quality standards. We do this through external accreditation and auditing, as well as internal practices and performance monitoring.
- Accreditations
- Systems & Procedures
- Practices
- Continuous Improvement
- REACH Statement
- COVID-19 Statement
- Suppliers
- Parafix is proud to partner with world leading tape and material manufacturers. With access to a broad range of products we offer our customers extensive choice and the opportunity to combine complimentary materials from any manufacturer, unique to their requirements.
- 3M
- Rogers Corporation
- Porex
- Pregis
- Adhesives Research
- Scapa
- Tesa
- Laird
- Vancive
- Pall Corporation
- Advance Tapes
- Avery Dennison
- DuPont
- Freudenberg
- Furukawa
- Nitto
- Amcor
- Our Culture
- We are proud of our ‘can do’ attitude at Parafix, always striving to solve our customers’ challenges. We are also committed to quality and aim to treat our customers, employees and communities fairly and with care.
- Our Values
- Our company values ensure we add value to and maintain the high levels of service throughout all of our activities. Read our company values on this page.
- History
- In 1969 we started our journey as a small distributor of adhesive tape. Today we’ve become a leading converter in Europe, creating complex adhesive components used globally.
- Parafix in the Community
- One of our company values is to support the local communities in which we operate. This page shares some of the community activities we engage in and good causes we offer our support to.
- Careers
- Browse through the job vacancies in the Lancing, Sussex.
- Why Parafix
- Resources
- Why Use Tape?
- There are many reasons why tapes are replacing traditional fixing methods. Among these is the strong and reliable bond that tapes provide and their additional functionality such as sealing, shielding, insulating and cushioning. In addition to these benefits, joining with tape offers greater design options, product quality and enhanced manufacturing efficiencies.
- Why Customise Tape?
- A customised adhesive component offers a number of benefits to designers and engineers. Product designs can be streamlined with invisible joins and product manufacture is made simple with a convenient and easy to handle component. Adhesive parts can be designed with application aids, or for use in automated assembly processes.
- Tape vs Traditional Fixings
- Tape is replacing traditional fixing methods across many applications. Here we compare the benefits of using a tape in place of traditional fixing methods.
- Tape vs. Spot Welds
- Tape vs. Clips
- Tape vs. Liquid Adhesive
- Tape vs. Mechanical Fastenings
- Choosing Your Material
- When choosing an adhesive product, there are some key criteria to consider so that the desired performance is achieved. By understanding the end product and conditions it will be subject to, a material can be specified that will last the full life of your product.
- Substrates
- Bond Stress
- Environmental Conditions
- End Product
- Foam bonding
- Using Your Material
- The way you use your tape is an important factor in ensuring the desired performance is achieved. We’ve put together some tips on looking after your material and applying it correctly.
- Storing Your Material
- Surface Preparation
- Manual Application
- Automated Application
- News
- For all of the latest on Parafix activities, product innovations and industry updates.
- Videos
- Browse through our videos to learn more about Parafix, our products and our many applications.
- Case Studies
- Learn about some of the projects we've worked on.
- Downloads
- Browse through our selection of product and industry specific downloadable documents.
- FAQs
- Answers to some of our most frequently asked questions.
- Glossary
- Explanations of commonly used terminology in the tape converting industry.
- Wishlist
- Store a list of products for sharing or requesting additional information, pricing and samples.
- Why Use Tape?
- Contact
Development & manufacture of biosensor components
- ParafixCase StudiesBondingDevelopment & manufacture of biosensor components
-
Intricate and tight tolerance biosensor components
Our customer required assistance in the development of a complex, high volume biosensor device that required tight tolerances and fast, very accurate assembly.
Parafix were heavily involved in the design and development of the biosensor components using laser, plotter and tooled development parts through our R&D Department.
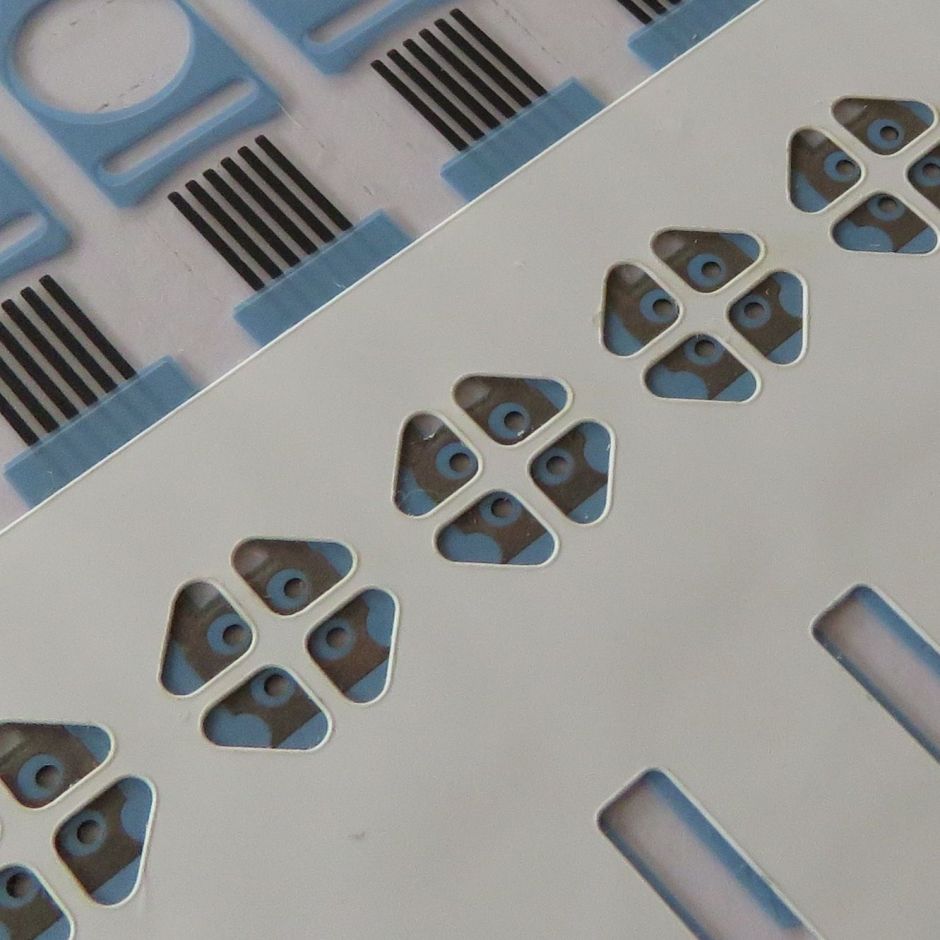
Optimum designs and materials were determined, resulting in the production of five components with very specific functions, including a flow cell, made with an inert diagnostic spacer tape, a flow cell lid using a hydrophilic film, a microporous mesh for air flow and barrier to liquid, a component for recognition in vision systems and a PET backing to provide stiffness and enhance aesthetics.
We identified suitable materials and developed the biosensor components and assembly process, incorporating our automated assembly equipment for quick and very accurate assembly.
Read about this project in more detail below.
-
- Design components for biosensor to channel blood through very thin capillary channels, to simultaneously supply four wells from one drop of blood.
- The channels had to be very thin so as not to lose too much of the sample transported to the wells and to reduce the amount of blood required from the patient.
- Fluids must not escape
- Oxygen is required for the test
- The gas from the reaction needs to escape
- Must be manufactured in a controlled environment
- Must be manufactured to ISO 13485
- An automated assembly process is required due to the required speed and accuracy of assembly.
- In conjunction with the customer, Parafix were heavily involved in the design and development of the part and material selection, providing a number of designs and prototypes to trial, for example experimenting with many channel designs to find the perfect performing design.
- We designed five components for the biosensor part, assembled with our AccuPlace automated placement equipment.
- Component one was the flow cell, made with an inert diagnostic spacer tape. The thin capillary channels and wells required laser cutting as they were just 200um wide. We developed our own laser system to cut the channels and small holes in volume.
- Component two was a lid for the flow cell, made with a hydrophilic film.
- Component three was a microporous mesh placed underneath the flow cell, which provided a barrier to fluids, whilst allowing oxygen in for the test to work, and the gas from the reaction to escape.
- Component four was a black double coated tape to allow inspection and provide a contrast in the part for recognition by the vision systems of our AccuPlace automated placement equipment.
- Component five was a thick, white PET backing to provide stiffness and to improve the aesthetics of the part.
- All components were manufactured and assembled in our ISO 14001 class 7 clean room and we are accredited with ISO 13485.
- The assembly process was developed and we supplied the fully automatic assembly equipment to accurately assemble all components to a target speed.
- Using our knowledge and access to a wide range of medical grade materials, the optimum products were specified for the diagnostic device.
- The thoroughly tested component was supplied in a presentation suitable for the automated assembly system.
- The assembly process was tried and tested during the design and prototyping stage and Parafix supplied a complete automated assembly system and components, installed and ready to operate at the customer’s manufacturing facility.